Thermodynamics problems
- 1. One m3 of methane (CH4) requires 2m3 of oxygen and produces 1m3 of carbon dioxide and 2m3 of water or steam.
Options- A. Correct
- B. Incorrect Discuss
Correct Answer: Correct
- 2. The variables which control the physical properties of a perfect gas are
Options- A. pressure exerted by the gas
- B. volume occupied by the gas
- C. temperature of the gas
- D. all of these Discuss
Correct Answer: all of these
- 3. The reading of the pressure gauge fitted on a vessel is 25 bar. The atmospheric pressure is 1.03 bar and the value of 'g' is 9.81 m/s2. The absolute pressure in the vessel is
Options- A. 23.97 bar
- B. 25 bar
- C. 26.03 bar
- D. 34.81 bar Discuss
Correct Answer: 26.03 bar
- 4. Select the wrong statement
Options- A. A Joule cycle consists of two constant volume and two isentropic processes.
- B. An Otto cycle consists of two constant volume and two isentropic processes.
- C. An Ericsson cycle consists of two constant pressure and two isothermal processes.
- D. all of the above Discuss
Correct Answer: A Joule cycle consists of two constant volume and two isentropic processes.
- 5. One kg of hydrogen requires 8 kg of oxygen and produces
Options- A. 1 kg of water
- B. 7 kg of water
- C. 8 kg of water
- D. 9 kg of water Discuss
Correct Answer: 9 kg of water
- 6. The absolute zero pressure will be
Options- A. when molecular momentum of the system becomes zero
- B. at sea level
- C. at the temperature of - 273 K
- D. at the centre of the earth Discuss
Correct Answer: when molecular momentum of the system becomes zero
- 7. In an extensive property of a thermodynamic system
Options- A. extensive heat is transferred
- B. extensive work is done
- C. extensive energy is utilised
- D. none of these Discuss
Correct Answer: none of these
- 8. An open system is one in which
Options- A. heat and work crosses the boundary of the system, but the mass of the working substance does not crosses the boundary of the system
- B. mass of the working substance crosses the boundary of the system but the heat and work does not crosses the boundary of the system
- C. both the heat and work as well as mass of the working substance crosses the boundary of the system
- D. neither the heat and work nor the mass of the working substance crosses the boundary of the system Discuss
Correct Answer: both the heat and work as well as mass of the working substance crosses the boundary of the system
- 9. Workdone during adiabatic expansion is given by (where p1 v1, T1 = Pressure, volume and temperature for the initial condition of gas, p2, v2, T2 = Corresponding values for the final condition of gas, R = Gas constant, and ? = Ratio of specific heats)
Options- A.
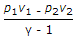
- B.
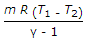
- C.
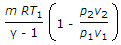
- D. all of these Discuss
Correct Answer: all of these
- 10. When the gas is cooled at constant pressure,
Options- A. its temperature increases but volume decreases
- B. its volume increases but temperature decreases
- C. both temperature and volume increases
- D. both temperature and volume decreases Discuss
Correct Answer: both temperature and volume decreases
More in Mechanical Engineering:
Programming
Copyright ©CuriousTab. All rights reserved.
