Stoichiometry problems
- 1. Which equation is not an equation of state?
Options- A. PV = RT + B/V + y/V2 + ....
- B. (P + a/V2)(V-b) = RT
- C.
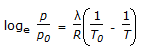
- D.
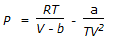 Discuss
Discuss
Correct Answer:
- 2. One kg of saturated steam at 100°C and 1.01325 bar is contained in a rigid walled vessel. It lias a volume of 1.673 m3. It cools to 98°C ; the saturation pressure is 0.943 bar ; one kg of water vapour under these conditions has a volume of 1.789 m3. The latent heat of condensation (kJ/kg-1 ) under these conditions is
Options- A. 40732
- B. 2676
- C. 2263
- D. 540 Discuss
Correct Answer: 2263
- 3. Which of the following is not a unit of kinematic viscosity?
Options- A. Poise
- B. Stoke
- C. cm2/second
- D. None of these Discuss
Correct Answer: Poise
- 4. 1 gm mole of methane (CH4) contains
Options- A. 6.02 x 1023 atoms of hydrogen.
- B. 4 gm atoms of hydrogen.
- C. 3.01 x 1023 molecules of methane.
- D. 3 gms of carbon. Discuss
Correct Answer: 4 gm atoms of hydrogen.
- 5. Percentage saturation of a vapor bearing gas is always __________ the relative saturation.
Options- A. higher than
- B. smaller than
- C. equal to
- D. either (a) or (b); depends on the system Discuss
Correct Answer: smaller than
- 6. The vapor pressures of benzene and toluene are 3 and 4/3 atmospheres respectively. A liquid feed of 0.4 moles of benzene and 0.6 moles of toluene is vapourised. Assuming that the products are in equilibrium, the vapor phase mole fraction of benzene is
Options- A. 0.4
- B. 0.6
- C. 0.8
- D. 0.2 Discuss
Correct Answer: 0.6
- 7. Ideal solution is formed, when its components have zero
Options- A. heat of mixing.
- B. volume change.
- C. both (a) & (b).
- D. neither (a) nor (b). Discuss
Correct Answer: both (a) & (b).
- 8. Enthalpy of formation of NH3 is - 46 kJ/kg mole. The enthalpy change for the gaseous reaction, 2NH3 ? N2 + 3H2, is equal to __________ kJ/kg. mole.
Options- A. 46
- B. 92
- C. -23
- D. -92 Discuss
Correct Answer: 92
- 9. Which of the following holds good for a solution obeying Raoult's law (i.e., an ideal solution) (where, ?H = heat of mixing, and ?V = volume change on mixing )?
Options- A. ?H = 1 (+ ve)and ? V = -ve
- B. ?H = 0
- C. ?V = 0
- D. both (b) and (c) Discuss
Correct Answer: both (b) and (c)
- 10. Pick out the wrong unit conversion of mass transfer co-efficient.
Options- A. 1 lb/hr.ft3.atm. = 4.8182 kg/hr.m2.bar
- B. 1 kg/hr.m2.atm= 0.98687 kg/hr. m .bar
- C. 1 lb/hr . ft2 = 4.8823 kg/hr . m2
- D. 1 kg/hr . m2 = 4.8823 lb/hr . ft2 Discuss
Correct Answer: 1 kg/hr . m2 = 4.8823 lb/hr . ft2
More in Chemical Engineering:
Programming
Copyright ©CuriousTab. All rights reserved.
