Chemical Engineering Thermodynamics problems
- 1. 1st law of thermodynamics is nothing but the law of conservation of
Options- A. momentum
- B. mass
- C. energy
- D. none of these Discuss
Correct Answer: energy
- 2. Equilibrium constant decreases as the temperature
Options- A. increases, for an exothermic reaction.
- B. decreases, for an exothermic reaction.
- C. increases, for an endothermic reaction.
- D. none of these. Discuss
Correct Answer: increases, for an exothermic reaction.
- 3. Which of the following is a thermodynamic property of a system?
Options- A. Concentration
- B. Mass
- C. Temperature
- D. Entropy Discuss
Correct Answer: Entropy
- 4. Heating of water under atmospheric pressure is an __________ process.
Options- A. isochoric
- B. isobaric
- C. adiabatic
- D. isothermal Discuss
Correct Answer: isobaric
- 5. Solubility of a substance which dissolves with an increase in volume and liberation of heat will be favoured by the
Options- A. low pressure and high temperature.
- B. low pressure and low temperature.
- C. high pressure and low temperature.
- D. high pressure and high temperature. Discuss
Correct Answer: low pressure and low temperature.
- 6. The chemical potential of a component (?i) of a phase is the amount by which its capacity for doing all work, barring work of expansion is increased per unit amount of sustance added for an infinitesimal addition at constant temperature and pressure. It is given by
Options- A.
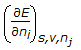
- B.
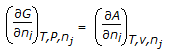
- C.
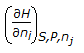
- D. all (a), (b) and (c) Discuss
Correct Answer: all (a), (b) and (c)
- 7. In a working refrigerator, the value of COP is always
Options- A. 0
- B. < 0
- C. < 1
- D. > 1 Discuss
Correct Answer: > 1
- 8. Linde gas liquefaction process employs cooling
Options- A. by throttling
- B. by expansion in an engine
- C. at constant pressure
- D. none of these Discuss
Correct Answer: by throttling
- 9. The theoretical minimum work required to separate one mole of a liquid mixture at 1 atm, containing 50 mole % each of n- heptane and n- octane into pure compounds each at 1 atm is
Options- A. -2 RT ln 0.5
- B. -RT ln 0.5
- C. 0.5 RT
- D. 2 RT Discuss
Correct Answer: -RT ln 0.5
- 10. A chemical reaction will occur spontaneously at constant pressure and temperature, if the free energy is
Options- A. zero
- B. positive
- C. negative
- D. none of these Discuss
Correct Answer: negative
More in Chemical Engineering:
Programming
Copyright ©CuriousTab. All rights reserved.
