Stoichiometry problems
- 1. 1 BTU/lb.?░F is equivalent to __________ kcal/kg.░C.
Options- A. 1
- B. 2.42
- C. 1.987
- D. 4.97 Discuss
Correct Answer: 1
- 2. 1 gm mole of an alcohol whose molecular weight is 74 contains 48 gms of carbon, 10 gms of hydrogen and 16 gms of oxygen. Its molecular formula is
Options- A. C4H9OH
- B. C3H21OH
- C. (C2H4)2H2.OH
- D. C2H33OH Discuss
Correct Answer: C4H9OH
- 3. The average translational kinetic energy with which a gas molecule is endowed is dependent on its
Options- A. nature
- B. size
- C. absolute temperature
- D. all (a), (b) & (c) Discuss
Correct Answer: absolute temperature
- 4. The reaction A + B ? C has been conducted in a reactor as shown below.
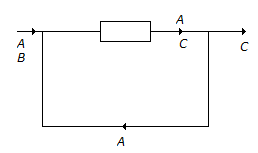
The number of boundaries around which material balance can be written, are
Options- A. 1
- B. 6
- C. 3
- D. 4 Discuss
Correct Answer: 4
- 5. Osmotic pressure of the solution can be increased by
Options- A. decreasing its temperature.
- B. increasing the volume of the vessel containing the solution.
- C. diluting the solution.
- D. none of these. Discuss
Correct Answer: none of these.
- 6. Concentration of a solution expressed in terms of __________ is independent of temperature.
Options- A. molarity
- B. normality
- C. molality
- D. none of these Discuss
Correct Answer: molality
- 7. 1 kg/m2 is equal to __________ mm water column.
Options- A. 1
- B. 10
- C. 100
- D. 1000 Discuss
Correct Answer: 1
- 8. One micron is equal to __________ cm.
Options- A. 10-2
- B. 10-4
- C. 10-6
- D. 10-8 Discuss
Correct Answer: 10-4
- 9. A vapor that exists above its critical temperature is termed as a __________ vapor.
Options- A. saturated
- B. unsaturated
- C. gaseous
- D. sub-cooled Discuss
Correct Answer: gaseous
- 10. The value of the gas-law constant 'R' is 1.987
Options- A. kcal/kg-mole.░C
- B. Btu/lb-mole.░R
- C. kcal/kg-mole.░K
- D. both (b)& (c) Discuss
Correct Answer: both (b)& (c)
More in Chemical Engineering:
Programming
Copyright ęCuriousTab. All rights reserved.
