Stoichiometry problems
- 1. The heat of solution depends upon the
Options- A. nature of solvent
- B. concentration of solution
- C. nature of solute
- D. all (a), (b) & (c) Discuss
Correct Answer: all (a), (b) & (c)
- 2. "The equilibrium value of the mole fraction of the gas dissolved in a liquid is directly proportional to the partial pressure of that gas above the liquid surface". This statement pertaining to the solubility of gases in liquid is the __________ law.
Options- A. Raoult's
- B. Henry's
- C. Amgat's
- D. none of these Discuss
Correct Answer: Henry's
- 3. One mole of methane undergoes complete combustion in a stoichiometric amount of air. The reaction proceeds as CH4 + 2O2 ? CO2 + 2H2O. Both the reactants and products are in gas phase. ?H°298 = - 730 kJ/mole of methane. If the average specific heat of all the gases/vapour is 40 J/mole.K, the maximum temperature rise of the exhaust gases in °C would be approximately equal to
Options- A. 1225
- B. 1335
- C. 1525
- D. 1735 Discuss
Correct Answer: 1735
- 4. pH value of a solution containing 1 gm of hydrogen ion per litre will be
Options- A. 0
- B. 1
- C. 7
- D. 10 Discuss
Correct Answer: 0
- 5. 80 kg of Na2SO4 (molecular weight = 142) is present in 330 kg of an aqueous solution. The solution is cooled such that. 80 kg of Na2SO4 .10H2O crystals separate out. The weight fraction of Na2SO4 in the remaining solution is
Options- A. 0.00
- B. 0.18
- C. 0.24
- D. 1.00 Discuss
Correct Answer: 0.18
- 6. A flowsheet is given in the following figure:
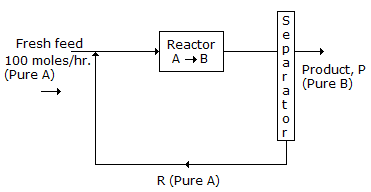
If the single pass once through conversion of A to B is 20%, then the rate of recycle R (molds/hr) is
Options- A. 300
- B. 400
- C. 500
- D. 600 Discuss
Correct Answer: 500
- 7. The temperature at which real gases obey the ideal gas law over a wide range of pressure is called the __________ temperature.
Options- A. reduced
- B. Boyle
- C. critical
- D. inversion Discuss
Correct Answer: inversion
- 8. What fraction of the total pressure is exerted by oxygen, if equal weights of oxygen and methane are mixed in an empty vessel at 25°C?
Options- A. 2/3
- B. 1/3
- C. 1/2
- D. l/3 x
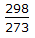 Discuss
Discuss
Correct Answer: 1/3
- 9. Claussius Clapeyron equation applies to the __________ process.
Options- A. sublimation
- B. melting
- C. vaporisation
- D. all (a), (b) & (c) Discuss
Correct Answer: all (a), (b) & (c)
- 10. As per Kirchoff s equation, the heat of reaction is affected by the
Options- A. pressure
- B. volume
- C. temperature
- D. molecularity Discuss
Correct Answer: temperature
More in Chemical Engineering:
Programming
Copyright ©CuriousTab. All rights reserved.
