Stoichiometry problems
- 1. Variation of vapor pressure with temperature can be calculated using Clausius-Clapeyron equation, which assumes that the
Options- A. vapor follows the ideal gas law.
- B. molal latent heat of vaporisation is constant within the limited temperature range.
- C. volume in the liquid state is negligible compared with that in the vapor state.
- D. all (a), (b) & (c). Discuss
Correct Answer: all (a), (b) & (c).
- 2. Methane is mixed with stoichiometric proportion of oxygen and completely combusted. The number of additional specifications required to determine the product flow rate and composition is
Options- A. 0
- B. 1
- C. 2
- D. 3 Discuss
Correct Answer: 0
- 3. If the pH value of a solution changes by one unit, it implies that hydrogen ion concentration in the solution will change __________ times.
Options- A. 10
- B. 20
- C. 70
- D. 100 Discuss
Correct Answer: 10
- 4. With rise in temperature, the solubility of ammonia in water at a fixed pressure
Options- A. increases
- B. decreases
- C. remains unchanged
- D. increases exponentially Discuss
Correct Answer: decreases
- 5. Avogadro's number is equal to
Options- A. 6.023 x 1023 molecules/kg.mole.
- B. 6.023 x 1023 molecules/gm.mole.
- C. 6.023 x 1016 molecules/kg.mole.
- D. 6.023 x 1026 molecules/gm.mole. Discuss
Correct Answer: 6.023 x 1023 molecules/gm.mole.
- 6. Internal energy is independent of the __________ for an ideal gas.
Options- A. pressure
- B. volume
- C. both (a) & (b)
- D. neither (a) nor (b) Discuss
Correct Answer: both (a) & (b)
- 7. Which of the following is the Claussius-Clayperon equation?
Options- A. PV = RT + B/V + y/V2 + ....
- B. (P + a/V2)(V-b) = RT
- C.
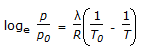
- D.
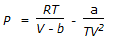 Discuss
Discuss
Correct Answer:
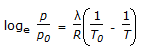
- 8. Unit of power is
Options- A. joule
- B. watt
- C. joule/Second
- D. both(b)&(c) Discuss
Correct Answer: both(b)&(c)
- 9. How many phases are present at eutectic point?
Options- A. 1
- B. 2
- C. 3
- D. unpredictable Discuss
Correct Answer: 3
- 10. Which of the following is insensitive to changes in pressure?
Options- A. Heat of vaporisation
- B. Melting point
- C. Heat of fusion
- D. Both (b) and (c) Discuss
Correct Answer: Both (b) and (c)
More in Chemical Engineering:
Programming
Copyright ©CuriousTab. All rights reserved.
