Thermodynamics problems
- 1. According to Kelvin-Planck's statement of second law of thermodynamics,
Options- A. it is impossible to construct an engine working on a cyclic process, whose sole purpose is to convert heat energy into work
- B. it is possible to construct an engine working on a cyclic process, whose sole purpose is to convert heat energy into work
- C. it is impossible to construct a device which operates in a cyclic process and produces no effect other than the transfer of heat from a cold body to a hot body
- D. none of the above Discuss
Correct Answer: it is impossible to construct an engine working on a cyclic process, whose sole purpose is to convert heat energy into work
- 2. The area under the temperature-entropy curve (T - s curve) of any thermodynamic process represents
Options- A. heat absorbed
- B. heat rejected
- C. either (a) or (b)
- D. none of these Discuss
Correct Answer: either (a) or (b)
- 3. For a perfect gas, according to Boyle's law (where p = Absolute pressure, v = Volume, and T = Absolute temperature)
Options- A. p v = constant, if T is kept constant
- B. v/T = constant, if p is kept constant
- C. p/T = constant, if v is kept constant
- D. T/p = constant, if v is kept constant Discuss
Correct Answer: p v = constant, if T is kept constant
- 4. According to Gay-Lussac law, the absolute pressure of a given mass of a perfect gas varies __________ as its absolute temperature, when the volume remains constant.
Options- A. directly
- B. indirectly Discuss
Correct Answer: directly
- 5. The efficiency of Diesel cycle depends upon
Options- A. temperature limits
- B. pressure ratio
- C. compression ratio
- D. cut-off ratio and compression ratio Discuss
Correct Answer: cut-off ratio and compression ratio
- 6. The general gas energy equation is (where Q1 - 2 = Heat supplied, dU = Change in internal energy, and W1 - 2 = Work done in heat units)
Options- A. Q1 - 2 = dU + W1 - 2
- B. Q1 - 2 = dU - W1 - 2
- C. Q1 - 2 = dU/W1 - 2
- D. Q1 - 2 = dU x W1 - 2 Discuss
Correct Answer: Q1 - 2 = dU + W1 - 2
- 7. The dual combustion cycle consists of one constant pressure, two constant volume and two isentropic processes.
Options- A. Agree
- B. Disagree Discuss
Correct Answer: Agree
- 8. When two bodies are in thermal equilibrium with a third body, they are also in thermal equilibrium with each other. This statement is called
Options- A. Zeroth law of thermodynamics
- B. First law of thermodynamics
- C. Second law of thermodynamics
- D. Kelvin Planck's law Discuss
Correct Answer: Zeroth law of thermodynamics
- 9. Kelvin-Planck's law deals with
Options- A. conservation of work
- B. conservation of heat
- C. conversion of heat into work
- D. conversion of work into heat Discuss
Correct Answer: conversion of heat into work
- 10. The expansion ratio (r) is the ratio of (where v1 = Volume at the beginning of expansion, and v2 = Volume at the end of expansion)
Options- A.
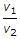
- B.
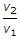
- C.
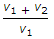
- D.
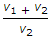 Discuss
Discuss
Correct Answer:
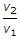
More in Mechanical Engineering:
Programming
Copyright ©CuriousTab. All rights reserved.
