Thermodynamics problems
- 1. One Joule (J) is equal to
Options- A. 1 N-m
- B. 1 kN-m
- C. 10 N-m/s
- D. 10 kN-m/s Discuss
Correct Answer: 1 N-m
- 2. The amount of heat given out by the complete combustion of 1 kg of fuel is known as calorific value of solid or liquid fuel.
Options- A. True
- B. False Discuss
Correct Answer: True
- 3. The volumetric or molar specific heat at constant pressure is the product of
Options- A. molecular mass of the gas and the specific heat at constant volume
- B. atomic mass of the gas and the gas constant
- C. molecular mass of the gas and the gas constant
- D. none of the above Discuss
Correct Answer: none of the above
- 4. The root mean square velocity of the gas molecules is given by (where k = Boltzmann's constant, T = Absolute temperature, and m = Mass of one molecule of a gas)
Options- A.
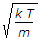
- B.
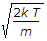
- C.
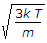
- D.
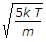 Discuss
Discuss
Correct Answer:
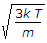
- 5. The area under the temperature-entropy curve (i. e. T - s curve) of any thermodynamic process represents the workdone during the process.
Options- A. Agree
- B. Disagree Discuss
Correct Answer: Disagree
- 6. Mond gas is obtained by
Options- A. partial combusion of coal, coke, anthracite coal or charcoal in a mixed air steam blast
- B. carbonisation of bituminous coal
- C. passing steam over incandescent coke
- D. passing air and a large amount of steam over waste coal at about 650°C Discuss
Correct Answer: passing air and a large amount of steam over waste coal at about 650°C
- 7. The heat and mechanical energies are mutually convertible. This statement was established by
Options- A. Boyle
- B. Charles
- C. Joule
- D. none of these Discuss
Correct Answer: Joule
- 8. The change of entropy, when heat is removed from the gas, is negative.
Options- A. Yes
- B. No Discuss
Correct Answer: Yes
- 9. A triatomic molecule consists of __________ atoms.
Options- A. one
- B. two
- C. three
- D. four Discuss
Correct Answer: three
- 10. One molecule of oxygen consists of __________ atoms of oxygen.
Options- A. 2
- B. 4
- C. 8
- D. 16 Discuss
Correct Answer: 2
More in Mechanical Engineering:
Programming
Copyright ©CuriousTab. All rights reserved.
