Discussion
Home ‣ Chemical Engineering ‣ Chemical Engineering Thermodynamics Comments
- Question
Boyle's law for gases states that
Options- A.
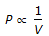 , when temperature is constant.
, when temperature is constant. - B.
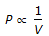 , when temperature & mass of the gas remain constant.
, when temperature & mass of the gas remain constant. - C. P ? V, at constant temperature & mass of the gas.
- D.
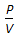 = constant, for any gas.
= constant, for any gas. - Correct Answer
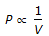 , when temperature & mass of the gas remain constant.
, when temperature & mass of the gas remain constant.
- 1. "At the absolute zero temperature, the entropy of every perfectly crystalline substance becomes zero". This follows from the
Options- A. third law of thermodynamics
- B. second law of thermodynamics
- C. Nernst heat theorem
- D. Maxwell's relations Discuss
- 2. Which of the following is not an intensive property?
Options- A. Chemical potential
- B. Surface tension
- C. Heat capacity
- D. None of these Discuss
- 3. Van Laar equation deals with the activity co-efficients in
Options- A. binary solutions
- B. ternary solutions
- C. azeotropic mixture only
- D. none of these Discuss
- 4. For a stable phase at constant pressure and temperature, the fugacity of each component in a binary system __________ as its mole fraction increases.
Options- A. decreases
- B. increases
- C. remains same
- D. decreases linearly Discuss
- 5. In jet refrigerators, the refrigerating fluid is practically always
Options- A. water
- B. ammonia
- C. freon
- D. brine Discuss
- 6. For a spontaneous process, free energy
Options- A. is zero
- B. increases
- C. decreases whereas the entropy increases
- D. and entropy both decrease Discuss
- 7. Pick out the wrong statement pertaining to the decomposition of PCl5 represented by, PCl5 ⇌ PCl3 + Cl2.Degree of dissociation of PCl5 will
Options- A. decrease on addition of Cl2.
- B. increase on addition of an inert gas at constant pressure.
- C. decrease on increasing the pressure of the system.
- D. none of these Discuss
- 8. Pick out the correct statement:
Options- A. In an isothermal system, irreversible work is more than reversible work.
- B. Under reversible conditions, the adiabatic work is less than isothermal work.
- C. Heat, work, enthalpy and entropy are all 'state functions'.
- D. Matter and energy can not be exchanged with the surroundings in a closed system. Discuss
- 9. A refrigeration cycle is a reversed heat engine. Which of the following has the maximum value of the co-efficient of performance (COP) for a given refrigeration effect?
Options- A. Vapor compression cycle using expansion valve.
- B. Air refrigeration cycle.
- C. Vapor compression cycle using expansion engine.
- D. Carnot refrigeration cycle. Discuss
- 10. The accentric factor of a materical, '?', is defined as ? = -log10(Prsat)Tr-1 = 0.7, where, Prsat = reduced vapor pressure, Tr = reduced temperature. The value of accentric factor is always
Options- A. > 2
- B. < 1
- C. > 1
- D. < 3 Discuss
Chemical Engineering Thermodynamics problems
Search Results
Correct Answer: third law of thermodynamics
Correct Answer: Heat capacity
Correct Answer: binary solutions
Correct Answer: increases
Correct Answer: water
Correct Answer: decreases whereas the entropy increases
Correct Answer: none of these
Correct Answer: Under reversible conditions, the adiabatic work is less than isothermal work.
Correct Answer: Carnot refrigeration cycle.
Correct Answer: < 1
Comments
There are no comments.More in Chemical Engineering:
Programming
Copyright ©CuriousTab. All rights reserved.
