Discussion
Home ‣ Chemical Engineering ‣ Chemical Engineering Thermodynamics Comments
- Question
Which of the following is not an intensive property?
Options- A. Chemical potential
- B. Surface tension
- C. Heat capacity
- D. None of these
- Correct Answer
- Heat capacity
- 1. Van Laar equation deals with the activity co-efficients in
Options- A. binary solutions
- B. ternary solutions
- C. azeotropic mixture only
- D. none of these Discuss
- 2. For a stable phase at constant pressure and temperature, the fugacity of each component in a binary system __________ as its mole fraction increases.
Options- A. decreases
- B. increases
- C. remains same
- D. decreases linearly Discuss
- 3. In jet refrigerators, the refrigerating fluid is practically always
Options- A. water
- B. ammonia
- C. freon
- D. brine Discuss
- 4. The co-efficient of performance (COP) of a refrigerating system, which is its index of performance, is defined as the ratio of useful refrigeration to the net work. The units of __________ and COP are the same.
Options- A. kinematic viscosity
- B. work
- C. temperature
- D. none of these Discuss
- 5. The expression,
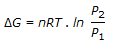 , gives the free energy change
, gives the free energy change
Options- A. with pressure changes at constant temperature.
- B. under reversible isothermal volume change.
- C. during heating of an ideal gas.
- D. during cooling of an ideal gas. Discuss
- 6. "At the absolute zero temperature, the entropy of every perfectly crystalline substance becomes zero". This follows from the
Options- A. third law of thermodynamics
- B. second law of thermodynamics
- C. Nernst heat theorem
- D. Maxwell's relations Discuss
- 7. Boyle's law for gases states that
Options- A.
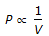 , when temperature is constant.
, when temperature is constant. - B.
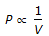 , when temperature & mass of the gas remain constant.
, when temperature & mass of the gas remain constant. - C. P ? V, at constant temperature & mass of the gas.
- D.
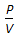 = constant, for any gas.
Discuss
= constant, for any gas.
Discuss
- 8. For a spontaneous process, free energy
Options- A. is zero
- B. increases
- C. decreases whereas the entropy increases
- D. and entropy both decrease Discuss
- 9. Pick out the wrong statement pertaining to the decomposition of PCl5 represented by, PCl5 ⇌ PCl3 + Cl2.Degree of dissociation of PCl5 will
Options- A. decrease on addition of Cl2.
- B. increase on addition of an inert gas at constant pressure.
- C. decrease on increasing the pressure of the system.
- D. none of these Discuss
- 10. Pick out the correct statement:
Options- A. In an isothermal system, irreversible work is more than reversible work.
- B. Under reversible conditions, the adiabatic work is less than isothermal work.
- C. Heat, work, enthalpy and entropy are all 'state functions'.
- D. Matter and energy can not be exchanged with the surroundings in a closed system. Discuss
Chemical Engineering Thermodynamics problems
Search Results
Correct Answer: binary solutions
Correct Answer: increases
Correct Answer: water
Correct Answer: none of these
Correct Answer: with pressure changes at constant temperature.
Correct Answer: third law of thermodynamics
Correct Answer:
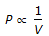 , when temperature & mass of the gas remain constant.
, when temperature & mass of the gas remain constant. Correct Answer: decreases whereas the entropy increases
Correct Answer: none of these
Correct Answer: Under reversible conditions, the adiabatic work is less than isothermal work.
Comments
There are no comments.More in Chemical Engineering:
Programming
Copyright ©CuriousTab. All rights reserved.
