Discussion
Home ‣ Chemical Engineering ‣ Stoichiometry Comments
- Question
The value of Trouton's ratio (?b/Tb) for a number of substances is 21 (where, ?b = molal that of vaporisation of a substance at its normal boiling point, KCal/kg. mole and Tb = normal boiling point, °K). The Kis-tyakowsky equation is used for calculation of Trouton's ratio of __________ liquids.
Options- A. polar
- B. non-polar
- C. both (a) & (b)
- D. neither (a) nor (b)
- Correct Answer
- both (a) & (b)
- 1. For a neutral solution (pH = 7), the value of[H+] [OH-] is equal to
Options- A. 0
- B. 1
- C. < 1
- D. > 1 Discuss
- 2. The vapor pressure of a substance, at its melting point, is __________ in the solid state as compared to that in the liquid state.
Options- A. less
- B. more
- C. same
- D. either (a) or (b); depends on the nature of the substance Discuss
- 3. Recycling in a chemical process facilitates
Options- A. increased yield
- B. enrichment of product
- C. heat conservation
- D. all (a), (b) &(c) Discuss
- 4. The value of the gas-law constant 'R' is 1.987
Options- A. kcal/kg-mole.°C
- B. Btu/lb-mole.°R
- C. kcal/kg-mole.°K
- D. both (b)& (c) Discuss
- 5. A vapor that exists above its critical temperature is termed as a __________ vapor.
Options- A. saturated
- B. unsaturated
- C. gaseous
- D. sub-cooled Discuss
- 6. The combustion equations of carbon and carbon monoxide are as follows:
C + O2 = CO2, ?H = - 394 kJ/kg . mole CO + 1/2 O2 = CO2, ?H = - 284.5 kJ/kg. mole.
The heat of formation of CO is __________ kJ/kg. mole.
Options- A. -109.5
- B. +109.5
- C. +180
- D. +100 Discuss
- 7. For any system, the __________ heat of solution is dependent on the temperature and the adsorbate concentration.
Options- A. integral
- B. differential
- C. both (a) & (b)
- D. neither (a) nor (b) Discuss
- 8. The optimum size ratio for two mixed reactors in series depends on the kinetics of the reaction and the conversion level. For reaction orders more than one, the
Options- A. equal sized reactors are the best.
- B. smaller reactor should come first.
- C. larger reactor should come first.
- D. none of these. Discuss
- 9.
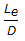 for fully open gate valves would be
for fully open gate valves would be
Options- A. much more than that for fully open globe valves.
- B. much less (say 2% than that for fully open globe valves).
- C. around 7.
- D. both (b) and (c). Discuss
- 10. Ponchon Savarit method is based on the use of enthalpy concentration diagram, which contains the bubble point curve (saturated liquid curve), dew point curve (saturated vapour curve) and equilibrium tie lines. As compared to McCabe-Thiele's method, this method
Options- A. is more accurate in finding the number of equilibrium stages.
- B. accounts for the enthalpy changes in the process.
- C. facilitates direct calculation of heat load on reboiler & condenser from, the diagram used in this method.
- D. all 'a', 'b' & 'c'. Discuss
Stoichiometry problems
Search Results
Correct Answer: 1
Correct Answer: same
Correct Answer: all (a), (b) &(c)
Correct Answer: both (b)& (c)
Correct Answer: gaseous
Correct Answer: -109.5
Correct Answer: both (a) & (b)
Correct Answer: smaller reactor should come first.
Correct Answer: both (b) and (c).
Correct Answer: all 'a', 'b' & 'c'.
Comments
There are no comments.More in Chemical Engineering:
Programming
Copyright ©CuriousTab. All rights reserved.
