Discussion
Home ‣ Chemical Engineering ‣ Stoichiometry See What Others Are Saying!
- Question
At what temperature, given mass of a gas that occupies a volume of 2 litres at N.T.P. will occupy a volume of 4 litres, if the pressure of the gas is kept constant?
Options- A. 273°C
- B. 273°K
- C. 100°C
- D. 200°C
- Correct Answer
- 273°C
- 1. Which of the following fractions of petroleum contains maximum sulphur?
Options- A. Diesel
- B. Gasoline
- C. Naphtha
- D. Atmospheric residue Discuss
- 2. Rate of heat release in a furnace, which is the measure of heat intensity is defined as
Options- A. kcal/hr/m3 combustion space.
- B. kcal/m3 combustion space.
- C. kcal/hr.
- D. none of these. Discuss
- 3. Paper pulp produced by kraft/sulphate process is
Options- A. bleached easily
- B. dull white in color
- C. strong fibrous
- D. dark colored Discuss
- 4. 1 torr is equivalent to
Options- A. 1 mm Hg
- B. 1 Pascal
- C. 1 ata
- D. 1 mm wc Discuss
- 5. An equation for calculating vapour pressure is given by, log10 P = A - B(t + c). This is called the
Options- A. Kistyakowsky equation
- B. Antonic equation
- C. Kopp's rule
- D. Trouton's rule Discuss
- 6. In continuous filtration (at a constant pressure drop), filtrate flow rate varies inversely as the
Options- A. square root of the velocity.
- B. square of the viscosity.
- C. filtration time only.
- D. washing time only. Discuss
- 7. Panel test determines the __________ of refractories.
Options- A. fusion point
- B. spalling resistance
- C. slag penetration resistance
- D. refractoriness under load (RUL) Discuss
- 8. Irradiation of water by ultraviolet light of suitable wavelength is commonly used for disinfection of water in
Options- A. food industry.
- B. municipal sewage treatment.
- C. petroleum refinery.
- D. iron & steel plant. Discuss
- 9. The density of a gas at N.T.P.is '?'. Keeping the pressure constant (i.e. 760 mm Hg), the 3 density of the gas will become
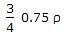 at a temperature of __________ °K
at a temperature of __________ °K
Options- A. 273°
- B. 300°
- C. 400°
- D. 300° Discuss
- 10. Pick out the wrong unit conversion of mass transfer co-efficient.
Options- A. 1 lb/hr.ft3.atm. = 4.8182 kg/hr.m2.bar
- B. 1 kg/hr.m2.atm= 0.98687 kg/hr. m .bar
- C. 1 lb/hr . ft2 = 4.8823 kg/hr . m2
- D. 1 kg/hr . m2 = 4.8823 lb/hr . ft2 Discuss
More questions
Correct Answer: Atmospheric residue
Correct Answer: kcal/hr/m3 combustion space.
Correct Answer: strong fibrous
Correct Answer: 1 mm Hg
Correct Answer: Antonic equation
Correct Answer: square root of the velocity.
Correct Answer: spalling resistance
Correct Answer: food industry.
Correct Answer: 400°
Correct Answer: 1 kg/hr . m2 = 4.8823 lb/hr . ft2
Comments
There are no comments.More in Chemical Engineering:
Programming
Copyright ©CuriousTab. All rights reserved.
