Discussion
Home ‣ Chemical Engineering ‣ Stoichiometry See What Others Are Saying!
- Question
The reaction A + B ? C has been conducted in a reactor as shown below.
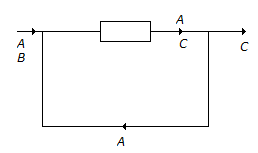
The number of balances (material) that can be made around the reactor are
Options- A. 1
- B. 2
- C. 3
- D. 4
- Correct Answer
- 3
- 1. Carbon/hydrogen ratio (by weight) is maximum (out of following) for
Options- A. gasoline
- B. kerosene
- C. light gas oil
- D. heavy fuel oil Discuss
- 2. Salt content in sea water is about __________ percent.
Options- A. 0.5
- B. 1
- C. 3.5
- D. 10 Discuss
- 3. Refractoriness/fusion points of 'superduty' refractories is __________ °C.
Options- A. 1520-1630
- B. 1630-1670
- C. > 1730
- D. > 2000 Discuss
- 4. Laminar flow of a Newtonion fluid ceases to exist, when the Reynolds number exceeds
Options- A. 4000
- B. 2100
- C. 1500
- D. 3000 Discuss
- 5. Plastic tubes & pipes are generally made by __________ moulding.
Options- A. injection
- B. transfer
- C. extrusion
- D. compression Discuss
- 6. pH value of soil is maintained at __________ by the addition of fertiliser for optimum growth and health of the plant.
Options- A. 4-5
- B. 7-8
- C. 9-10
- D. 12-13 Discuss
- 7. Sphericity of pulverised coal is
Options- A. 1
- B. <1
- C. >1
- D. ? Discuss
- 8. Coolant used in a boiling water reactor is
Options- A. hydrogen gas
- B. water
- C. steam
- D. a mixture of water & steam Discuss
- 9. Gasoline yield in catalytic reforming of naphtha may be about __________ percent by weight.
Options- A. 85
- B. 65
- C. 50
- D. 98 Discuss
- 10. Reciprocal of sphericity is termed as the
Options- A. specific surface ratio
- B. shape factor
- C. sauter diameter
- D. surface area per unit mass Discuss
More questions
Correct Answer: gasoline
Correct Answer: 3.5
Correct Answer: > 1730
Correct Answer: 2100
Correct Answer: extrusion
Correct Answer: 7-8
Correct Answer: <1
Correct Answer: a mixture of water & steam
Correct Answer: 85
Correct Answer: shape factor
Comments
There are no comments.More in Chemical Engineering:
Programming
Copyright ©CuriousTab. All rights reserved.
