Discussion
Home ‣ Chemical Engineering ‣ Stoichiometry See What Others Are Saying!
- Question
The molar composition of a gas is 10% H2, 10% O2, 30% CO2 and balance H2O. If 50% H2O condenses, the final mole percent of H2 in the gas on a dry basis will be
Options- A. 10%
- B. 5%
- C. 18.18%
- D. 20%
- Correct Answer
- 20%
- 1.
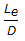 for a Tee (used as elbow, entering run) would be around
for a Tee (used as elbow, entering run) would be around
Options- A. 5
- B. 60
- C. 200
- D. 350 Discuss
- 2. 200 mesh seive size corresponds to __________ microns.
Options- A. 24
- B. 74
- C. 154
- D. 200 Discuss
- 3. Polycaprolactum is also known as
Options- A. nylon-66
- B. nylon-6
- C. teflon
- D. SBR Discuss
- 4. Carbon/hydrogen ratio (by weight) is maximum (out of following) for
Options- A. gasoline
- B. kerosene
- C. light gas oil
- D. heavy fuel oil Discuss
- 5. Salt content in sea water is about __________ percent.
Options- A. 0.5
- B. 1
- C. 3.5
- D. 10 Discuss
- 6. Refractoriness/fusion points of 'superduty' refractories is __________ °C.
Options- A. 1520-1630
- B. 1630-1670
- C. > 1730
- D. > 2000 Discuss
- 7. Laminar flow of a Newtonion fluid ceases to exist, when the Reynolds number exceeds
Options- A. 4000
- B. 2100
- C. 1500
- D. 3000 Discuss
- 8. Plastic tubes & pipes are generally made by __________ moulding.
Options- A. injection
- B. transfer
- C. extrusion
- D. compression Discuss
- 9. pH value of soil is maintained at __________ by the addition of fertiliser for optimum growth and health of the plant.
Options- A. 4-5
- B. 7-8
- C. 9-10
- D. 12-13 Discuss
- 10. Sphericity of pulverised coal is
Options- A. 1
- B. <1
- C. >1
- D. ? Discuss
More questions
Correct Answer: 60
Correct Answer: 74
Correct Answer: nylon-6
Correct Answer: gasoline
Correct Answer: 3.5
Correct Answer: > 1730
Correct Answer: 2100
Correct Answer: extrusion
Correct Answer: 7-8
Correct Answer: <1
Comments
There are no comments.More in Chemical Engineering:
Programming
Copyright ©CuriousTab. All rights reserved.
