Discussion
Home ‣ Chemical Engineering ‣ Stoichiometry See What Others Are Saying!
- Question
What fraction of the total pressure is exerted by oxygen, if equal weights of oxygen and methane are mixed in an empty vessel at 25°C?
Options- A. 2/3
- B. 1/3
- C. 1/2
- D. l/3 x
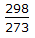
- Correct Answer
- 1/3
- 1. Pure aniline is evaporating through a stagnant air film of 1 mm thickness at 300 K and a total pressure of 100 KPa. The vapor pressure of aniline at 300 K is 0.1 KPa. The total molar concentration under these conditions is 40.1 mole/m3 . The diffusivity of aniline in air is 0.74xl0-5m2/s.The numerical value of mass transfer co-efficient is 7.4 x 10-3. The rate of evaporation of aniline is 2.97 x 10-4. Its units are
Options- A. mole/s
- B. mole/cm2. s
- C. mole/m2 . s
- D. kmole/m2 . s Discuss
- 2. The heat of adsorption of a gas caused by Van der Walls forces of attraction and capillarity is equal to the heat of
Options- A. normal condensation.
- B. wetting.
- C. sum of (a) and (b).
- D. difference of (a) and (b). Discuss
- 3. Under conditions of equal reduced pressure and equal reduced temperature, substances are said to be in the 'corresponding states'. At equal reduced conditions i.e., at the corresponding state, the __________ of different gases are nearly the same.
Options- A. compressibility
- B. molecular weight
- C. humidity
- D. none of these Discuss
- 4. In sweetening process, solutizer agent used with caustic alkali is
Options- A. potassium isobutyrate
- B. sodium plumbite
- C. methanol
- D. phenol Discuss
- 5. Rivets are generally specified by the
Options- A. head diameter
- B. shank diameter
- C. overall length
- D. none of these Discuss
- 6. In case of plain carbon steel, butt welded joints are used for shell plate thickness ? __________ cms.
Options- A. 1.2
- B. 0.5
- C. 3.8
- D. 6.8 Discuss
- 7. The main function of a muffle in the muffle furnace is to
Options- A. protect the charge from the effects of the products of combustion.
- B. smooth out temperature inequalities on the combustion side of the muffle wall.
- C. both (a) & (b)
- D. neither (a) nor (b) Discuss
- 8. Smoke point of a test sample of kerosene is found to be 15 mm. On removal of __________ from it, the smoke point rises to 25 mm.
Options- A. n-paraffins
- B. olefins
- C. aromatics
- D. none of these Discuss
- 9. Heating of orthophosphoric acid to 250°C produces
Options- A. metaphosphoric acid
- B. pyrophosphoric acid
- C. no change in it
- D. none of these Discuss
- 10. Total energy at a point comprises of __________ energy.
Options- A. potential & kinetic
- B. pressure
- C. internal
- D. all (a), (b) & (c). Discuss
More questions
Correct Answer: kmole/m2 . s
Correct Answer: sum of (a) and (b).
Correct Answer: compressibility
Correct Answer: potassium isobutyrate
Correct Answer: shank diameter
Correct Answer: 3.8
Correct Answer: both (a) & (b)
Correct Answer: aromatics
Correct Answer: pyrophosphoric acid
Correct Answer: all (a), (b) & (c).
Comments
There are no comments.More in Chemical Engineering:
Programming
Copyright ©CuriousTab. All rights reserved.
