Discussion
Home ‣ Chemical Engineering ‣ Stoichiometry See What Others Are Saying!
- Question
A 'limiting reactant' is the one, which decides the __________ in the chemical reacation.
Options- A. equilibrium constant
- B. conversion
- C. rate constant
- D. none of these
- Correct Answer
- conversion
- 1. Pick out the material having minimum Rittinger's number.
Options- A. Calcite
- B. Pyrite
- C. Quartz
- D. Galena Discuss
- 2. Average molecular weight of air is about
Options- A. 21
- B. 29
- C. 23
- D. 79 Discuss
- 3. Both tritium and deuterium have the same number of
Options- A. neutrons
- B. electrons
- C. protons
- D. nucleons Discuss
- 4. °API gravity of water at N.T.P. is about
Options- A. 0
- B. 1
- C. 10
- D. 100 Discuss
- 5. Carbon Content by weight in air dried wood may be about __________ percent.
Options- A. 10
- B. 25
- C. 50
- D. 80 Discuss
- 6. Energy produced in the nuclear fission is of the order of __________ MeV.
Options- A. 20
- B. 200
- C. 1000
- D. 2000 Discuss
- 7.
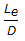 for a Tee (used as elbow, entering run) would be around
for a Tee (used as elbow, entering run) would be around
Options- A. 5
- B. 60
- C. 200
- D. 350 Discuss
- 8. 200 mesh seive size corresponds to __________ microns.
Options- A. 24
- B. 74
- C. 154
- D. 200 Discuss
- 9. Polycaprolactum is also known as
Options- A. nylon-66
- B. nylon-6
- C. teflon
- D. SBR Discuss
- 10. Carbon/hydrogen ratio (by weight) is maximum (out of following) for
Options- A. gasoline
- B. kerosene
- C. light gas oil
- D. heavy fuel oil Discuss
More questions
Correct Answer: Quartz
Correct Answer: 29
Correct Answer: neutrons
Correct Answer: 10
Correct Answer: 50
Correct Answer: 200
Correct Answer: 60
Correct Answer: 74
Correct Answer: nylon-6
Correct Answer: gasoline
Comments
There are no comments.More in Chemical Engineering:
Programming
Copyright ©CuriousTab. All rights reserved.
