Discussion
Home ‣ Chemical Engineering ‣ Stoichiometry See What Others Are Saying!
- Question
Pick out the wrong statement:
Options- A. Clausius-Clapeyron equation relates the latent heat of vaporisation to the slope of the vapor pressure curve.
- B. At the boiling point of liquid at the prevailing total pressure, saturated absolute humidity is infinite.
- C. Percentage saturation and relative saturation are numerically equal for an unsaturated vapor gas mixture.
- D. Clapeyron equation is given by,
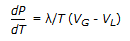 ; where, P = vapor pressure, T = absolute temperature, ? = latent heat of vaporisation, VG and VL = volumes of gas and liquid respectively.
; where, P = vapor pressure, T = absolute temperature, ? = latent heat of vaporisation, VG and VL = volumes of gas and liquid respectively. - Correct Answer
- Percentage saturation and relative saturation are numerically equal for an unsaturated vapor gas mixture.
- 1. Absorption factor is defined as (where, S1 = slope of the operating line S2 = slope of the equilibrium curve)
Options- A. S2/S1
- B. S1/S2
- C. S1 - S2
- D. S1 x S2 Discuss
- 2. Continuous shell temperature measurement in a liquid-liquid heat exchanger is done by a
Options- A. thermocouple
- B. reistance thermometer
- C. mercury in glass thermometer
- D. vapor pressure thermometer Discuss
- 3. Roof of a basic electric furnace is made of __________ bricks.
Options- A. superduty fireclay
- B. silica
- C. chromite
- D. none of these Discuss
- 4. Foot valves provided in pumps are __________ valves.
Options- A. relief
- B. three/four way
- C. pressure reducing
- D. directional control Discuss
- 5. Oxygen percentage in the flue gas coming out of a gaseous fuel fired furnace should be ideally about __________ percent.
Options- A. < 2
- B. < 5
- C. < 8
- D. < 10 Discuss
- 6. What is the Laplace transform of impulse input having magnitude 'X'?
Options- A. X
- B. X2
- C. 1/X
- D. 1 Discuss
- 7. Which of the following expressions defines the Baume gravity scale for liquids lighter than water?
Options- A. °Be = (140/G) - 130
- B. °Be = 200(G-1)
- C. °Be = 145 - (145/G)
- D. °Be = (400/G) - 400 Discuss
- 8. Which is not an acidic refractory?
Options- A. Silica
- B. Fireclay
- C. High alumina refractory
- D. Carbon black Discuss
- 9. Which of the following is the best nuclear fuel?
Options- A. Np- 239
- B. U-234
- C. Pu-239
- D. Th-236 Discuss
- 10. Sulphuric acid mist is arrested by using a __________ scrubber.
Options- A. packed wet
- B. hollow wet
- C. venturi
- D. co-current Discuss
More questions
Correct Answer: S1/S2
Correct Answer: thermocouple
Correct Answer: silica
Correct Answer: directional control
Correct Answer: < 2
Correct Answer: 1
Correct Answer: °Be = (140/G) - 130
Correct Answer: Carbon black
Correct Answer: Pu-239
Correct Answer: venturi
Comments
There are no comments.More in Chemical Engineering:
Programming
Copyright ©CuriousTab. All rights reserved.
