Discussion
Home ‣ Chemical Engineering ‣ Stoichiometry See What Others Are Saying!
- Question
Which of the following is the Claussius-Clayperon equation?
Options- A. PV = RT + B/V + y/V2 + ....
- B. (P + a/V2)(V-b) = RT
- C.
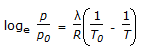
- D.
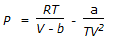
- Correct Answer
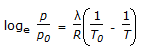
- 1. Pick out the correct statement.
Options- A. Deforestation helps in controlling the green house effect.
- B. Global warming is detrimental for increase in food productivity and may cause flood and cyclone.
- C. Lightening discharges are natural source of production of SO2 & H2S pollutant.
- D. Sulhur dioxide causes death by asphyxiation. Discuss
- 2. __________ atmosphere is maintained inside an iron blast furnace.
Options- A. Oxidising
- B. Reducing
- C. Inert
- D. Decarburising Discuss
- 3. The boiling points for pure water and pure toluene are 100°C and 110.6°C respectively. Toluene and water are completely immiscible in each other. A well agitated equimolar mixture of toluene and water are prepared. If, at a total pressure of one standard atm. exerted by the vapours of water and toluene, the mole fraction of water Xw in the vapour phase satisfies
Options- A. 0 < Xw < 0.5
- B. Xw = 0.5
- C. 0.5 < Xw < 1.0
- D. Xw = 1.0 Discuss
- 4. Condensation of bisphenol A with phosgene produces __________ which possess very good heat resistance.
Options- A. polyurathane
- B. polysulphone
- C. polycarbonate
- D. polyester Discuss
- 5. The vacuum maintained in vacuum distillation unit for reduced crude is about __________ mmHg.
Options- A. 1.2
- B. 12
- C. 120
- D. 700 Discuss
- 6. Buna-S is also called
Options- A. polyurathane
- B. SBR
- C. teflon
- D. bakelite Discuss
- 7. Dead zone in an instrument must be less than __________ percent of the scale.
Options- A. 0.2
- B. 1.5
- C. 4
- D. 8 Discuss
- 8. Fireclay bricks are used in the
Options- A. furnaces allowed to cool frequently
- B. flues
- C. chimney linings
- D. all (a), (b) and (c) Discuss
- 9. Aniline point of high speed diesel may be about __________ °C.
Options- A. 35
- B. 70
- C. 105
- D. 150 Discuss
- 10. Ebonite is a/an
Options- A. highly vulcanised rubber.
- B. natural rubber.
- C. unvulcanised raw rubber.
- D. adhesive. Discuss
More questions
Correct Answer: Global warming is detrimental for increase in food productivity and may cause flood and cyclone.
Correct Answer: Reducing
Correct Answer: 0.5 < Xw < 1.0
Correct Answer: polycarbonate
Correct Answer: 120
Correct Answer: SBR
Correct Answer: 0.2
Correct Answer: all (a), (b) and (c)
Correct Answer: 70
Correct Answer: highly vulcanised rubber.
Comments
There are no comments.More in Chemical Engineering:
Programming
Copyright ©CuriousTab. All rights reserved.
