Discussion
Home ‣ Mechanical Engineering ‣ Thermodynamics See What Others Are Saying!
- Question
The change of entropy, when heat is absorbed by the gas, is
Options- A. positive
- B. negative
- C. positive or negative
- Correct Answer
- positive
- 1. The flow in a pipe or channel is said to be non-uniform when
Options- A. the liquid particles at all sections have the same velocities
- B. the liquid particles at different sections have different velocities
- C. the quantity of liquid flowing per second is constant
- D. each liquid particle has a definite path Discuss
- 2. In water tube boilers
Options- A. water passes through the tubes which are surrounded by flames and hot gases
- B. the flames and hot gases pass through the tubes which are surrounded by water
- C. forced circulation takes place
- D. none of these Discuss
- 3. The cutting speed is maximum while machining __________ with a high speed steel tool.
Options- A. cast iron
- B. mild steel
- C. brass
- D. aluminium Discuss
- 4. The lip clearance angle is the angle formed by the
Options- A. leading edge of the land with a plane having the axis of the drill
- B. flank and a plane at right angles to the drill axis
- C. chisel edge and the lip as viewed from the end of a drill
- D. none of the above Discuss
- 5. The obtuse angle, included between the chisel edge and the lip as viewed from the end of a drill, is called
Options- A. helix or rake angle
- B. point angle
- C. chisel edge angle
- D. lip clearance angle Discuss
- 6. The carbide tools operating at very low cutting speeds (below 30 m/min)
Options- A. reduces tool life
- B. increases tool life
- C. have no effect on tool life
- D. spoils the work piece Discuss
- 7. Which of the following parameters govern the value of shear angle in continuous chip formation?
Options- A. True feed
- B. Chip thickness
- C. Rake angle of the cutting tool
- D. all of these Discuss
- 8. If u1 and u2 are the velocities of two moving bodies in the same direction before impact and v1 and v2 are their velocities after impact, then coefficient of restitution is given by
Options- A.
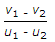
- B.
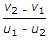
- C.
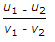
- D.
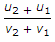 Discuss
Discuss
- 9. The heat transfer by conduction through a thick sphere is given by
Options- A.
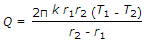
- B.
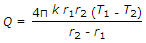
- C.
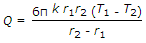
- D.
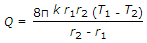 Discuss
Discuss
- 10. Nusselt number (NN) is given by
Options- A.
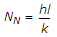
- B.
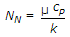
- C.
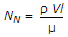
- D.
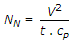 Discuss
Discuss
More questions
Correct Answer: the liquid particles at different sections have different velocities
Correct Answer: water passes through the tubes which are surrounded by flames and hot gases
Correct Answer: aluminium
Correct Answer: flank and a plane at right angles to the drill axis
Correct Answer: chisel edge angle
Correct Answer: reduces tool life
Correct Answer: all of these
Correct Answer:
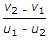
Correct Answer:
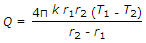
Correct Answer:
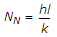
Comments
There are no comments.More in Mechanical Engineering:
Programming
Copyright ©CuriousTab. All rights reserved.
