Discussion
Home ‣ Mechanical Engineering ‣ Thermodynamics See What Others Are Saying!
- Question
A path 1-2-3 is given. A system absorbs 100 kJ as heat and does 60 kJ of work while along the path 1-4-3, it does 20 kJ of work. The heat absorbed during the cycle 1-4-3 is
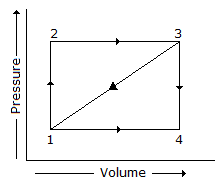
Options- A. -140 kJ
- B. -80 kJ
- C. -40 kJ
- D. +60 kJ
- Correct Answer
- +60 kJ
- 1. The specific weight is also known as weight density.
Options- A. Correct
- B. Incorrect Discuss
- 2. Which of the following material has maximum malleability?
Options- A. Lead
- B. Soft steel
- C. Wrought iron
- D. Copper Discuss
- 3. A petrol that detonates easily is called
Options- A. high octane petrol
- B. low octane petrol
- C. unleaded petrol
- D. blended fuel Discuss
- 4. A tool used to withdraw a drill from the sleeve is called
Options- A. drill remover
- B. drill puller
- C. drift
- D. drill drawer Discuss
- 5. The length of hacksaw blade is the distance between the outside edges of the holes which fits over the pins.
Options- A. Agree
- B. Disagree Discuss
- 6. Euler's formula holds good only for
Options- A. short columns
- B. long columns
- C. both short and long columns
- D. weak columns Discuss
- 7. Surface plate is used to check the trueness of flat surfaces.
Options- A. True
- B. False Discuss
- 8. Follow-up prescribes the sequence of operations to be followed.
Options- A. Correct
- B. Incorrect Discuss
- 9. An over-inflated tyre will wear the tread most near the
Options- A. edges
- B. corners
- C. centre
- D. none of these Discuss
- 10. A beam encastered at both the ends is called
Options- A. simply supported beam
- B. fixed beam
- C. cantilever beam
- D. continuous beam Discuss
More questions
Correct Answer: Correct
Correct Answer: Lead
Correct Answer: low octane petrol
Correct Answer: drift
Correct Answer: Agree
Correct Answer: long columns
Correct Answer: True
Correct Answer: Correct
Correct Answer: centre
Correct Answer: fixed beam
Comments
There are no comments.More in Mechanical Engineering:
Programming
Copyright ©CuriousTab. All rights reserved.
