Discussion
Home ‣ Mechanical Engineering ‣ Thermodynamics See What Others Are Saying!
- Question
Relation between cp and cv is given by (where cp = Specific heat at constant pressure, cv = Specific heat at constant volume, ? = cp/cv, known as adiabatic index, and R = Gas constant)
Options- A.
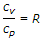
- B. cp - cv = R
- C.
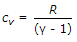
- D. Both (B) and (C)
- Correct Answer
- Both (B) and (C)
- 1. The work or surface speed for cylindrical grinding varies from
Options- A. 5 to 10 m/min
- B. 10 to 20 m/min
- C. 20 to 30 m/min
- D. 40 to 60 m/min Discuss
- 2. Ultra-sonic machining is best suited for
Options- A. tool steels
- B. sintered carbides
- C. glass
- D. all of these Discuss
- 3. In a plain milling cutter, the chip space between the back of one tooth and the face of the next tooth is called
Options- A. face
- B. fillet
- C. gash
- D. land Discuss
- 4. The silicon carbide abrasive is chiefly used for grinding
Options- A. cemented carbide
- B. ceramic
- C. cast iron
- D. all of these Discuss
- 5. Gears can be best produced on mass production by
Options- A. shaping
- B. casting
- C. forming
- D. hobbing Discuss
- 6. A thin cylindrical shell of diameter (d) length (l) and thickness (t) is subjected to an internal pressure (p). The longitudinal stress in the shell is
Options- A. pd/t
- B. pd/2t
- C. pd/4t
- D. pd/6t Discuss
- 7. The purpose of a thermostat in an engine cooling system is to
Options- A. prevent the coolant from boiling
- B. allow the engine to warm up quickly
- C. indicate the coolant temperature
- D. pressurise the system to raise the boiling point Discuss
- 8. In up milling, the thickness of chip is
Options- A. minimum at the beginning of the cut and maximum at the end of the cut
- B. maximum at the beginning of the cut and minimum at the end of the cut
- C. uniform throughout the cut
- D. none of these Discuss
- 9. In natural circulation steam boilers, the circulation of water is by convection currents which are set up during the heating of water.
Options- A. Correct
- B. Incorrect Discuss
- 10. In a coupling rod of a locomotive, each of the four pairs is a __________ pair.
Options- A. sliding
- B. turning
- C. rolling
- D. screw Discuss
More questions
Correct Answer: 20 to 30 m/min
Correct Answer: all of these
Correct Answer: gash
Correct Answer: all of these
Correct Answer: hobbing
Correct Answer: pd/4t
Correct Answer: allow the engine to warm up quickly
Correct Answer: minimum at the beginning of the cut and maximum at the end of the cut
Correct Answer: Correct
Correct Answer: turning
Comments
There are no comments.More in Mechanical Engineering:
Programming
Copyright ©CuriousTab. All rights reserved.
