Discussion
Home ‣ Chemical Engineering ‣ Chemical Reaction Engineering Comments
- Question
The fractional volume change of the system for the isothermal gas phase reaction, A → 3B , between no conversion and complete conversion is
Options- A. 0.5
- B. 1
- C. 2
- D. 3
- Correct Answer
- 2
- 1. A first order irreversible reaction, A → B is carried out separately in a constant volume as well as in a variable volume reactor for a particular period. It signifies that __________ in the two reactors.
Options- A. both conversion as well as concentration are same
- B. conversion in both will be the same but concentrations will be different
- C. both the conversion as well as concentrations will be different
- D. none of these. Discuss
- 2. For the irreversible elementary reactions in parallel viz
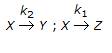 , the rate of disappearance of 'X' is equal to
, the rate of disappearance of 'X' is equal to
Options- A. CA(K1+K2)
- B. CA(K1 + K2)/2
- C. CA . K1/2
- D. CA . K2/2 Discuss
- 3. A catalyst
Options- A. initiates a reaction.
- B. lowers the activation energy of reacting molecules.
- C. is capable of reacting with any one of the reactants.
- D. can not be recovered chemically unchanged at the end of a chemical reaction. Discuss
- 4. Fractional conversion __________ with increase in pressure for ammonia synthesis reaction i.e., N2 + 3H2 ⇌ 2NH3.
Options- A. increases
- B. decreases
- C. remains unchanged
- D. unpredictable from the data Discuss
- 5. Rate of a gaseous phase reaction is given by,
 . The unit of rate constant is
. The unit of rate constant is
Options- A. (atm)-1
- B. (hr)-1
- C. (atm)-1.(hr)-1
- D. atm.(hr)-1 Discuss
- 6. For every 10°C rise in temperature, the rate of chemical reaction doubles. When the temperature is increased from 30 to 70°C, the rate of reaction increases __________ times.
Options- A. 8
- B. 12
- C. 16
- D. 32 Discuss
- 7. The increase in the rate of reaction with temperature is due to
Options- A. increase in the number of effective collisions.
- B. decrease in activation energy.
- C. increase in the average kinetic energy of the reacting molecules.
- D. none of these. Discuss
- 8. Cementite is
Options- A. Fe3C chemically.
- B. a compound of carbon and iron.
- C. characterised by an orthorhombic crystal structure.
- D. all (a), (b) and (c). Discuss
- 9. Refined acetic acid storage vessel are made of
Options- A. copper
- B. aluminium
- C. high carbon steel
- D. nickel Discuss
- 10. Which of the following crystal structures characterises the austenitic stainless steel?
Options- A. Simple hexagonal
- B. Body centred cubic
- C. Face centred cubic
- D. None of these Discuss
Chemical Reaction Engineering problems
Search Results
Correct Answer: conversion in both will be the same but concentrations will be different
Correct Answer: CA(K1+K2)
Correct Answer: lowers the activation energy of reacting molecules.
Correct Answer: increases
Correct Answer: (atm)-1.(hr)-1
Correct Answer: 16
Correct Answer: decrease in activation energy.
Correct Answer: all (a), (b) and (c).
Correct Answer: aluminium
Correct Answer: Face centred cubic
Comments
There are no comments.More in Chemical Engineering:
Programming
Copyright ©CuriousTab. All rights reserved.
