Discussion
Home ‣ Chemical Engineering ‣ Chemical Reaction Engineering Comments
- Question
Sulphuric acid is used as a catalyst in the
Options- A. hydrogenation of oils.
- B. gas phase oxidation of SO2 in chamber process.
- C. alkylation of hydrocarbons.
- D. none of these.
- Correct Answer
- alkylation of hydrocarbons.
- 1. Pick out the wrong statement.
Options- A. The vessel dispersion number (D/UL) for plug flow and mixed flow approaches zero and infinity respectively.
- B. Space time in a flow reactor is a measure of its capacity and is equal to the residence time when the density of reaction mixture is constant.
- C. Mixed reactor is always smaller than the plug flow reactor for all positive reaction orders for a particular duty.
- D. In an ideal tubular flow reactor, mixing takes place in radial direction and there is no mixing in logitudinal direction. Discuss
- 2. The most unsuitable reactor for carrying out reactions in which high reactant concentration favours high yields is
Options- A. backmix reactor
- B. plug flow reactor
- C. series of CSTR
- D. PFR in series Discuss
- 3. BET apparatus
Options- A. measures the catalyst surface area directly.
- B. operates at very high pressure.
- C. is made entirely of stainless steel.
- D. none of these. Discuss
- 4. Pick out the wrong statement pertaining to space velocity of flow reactors.
Options- A. The unit of space velocity is (time)-1 .
- B. The space velocity of 3 hr-1 means that three reactor volumes of feed at specified conditions are being fed into the reactor every hour.
- C. The space velocity of 3 hr-1 means that one third reactor volume of feed at specified conditions are being fed into the reactor.
- D. none of these. Discuss
- 5. Specific rate constant for a second order reaction
Options- A. is independent of temperature.
- B. varies with temperature.
- C. depends on the nature of the reactants.
- D. both (b) and (c). Discuss
- 6. A reactor is generally termed as an autoclave, when it is a
Options- A. high pressure batch reactor.
- B. atmospheric pressure tank reactor.
- C. high pressure tubular reactor.
- D. atmospheric pressure CSTR. Discuss
- 7. Rate of a gaseous phase reaction is given by,
 . The unit of rate constant is
. The unit of rate constant is
Options- A. (atm)-1
- B. (hr)-1
- C. (atm)-1.(hr)-1
- D. atm.(hr)-1 Discuss
- 8. Fractional conversion __________ with increase in pressure for ammonia synthesis reaction i.e., N2 + 3H2 ⇌ 2NH3.
Options- A. increases
- B. decreases
- C. remains unchanged
- D. unpredictable from the data Discuss
- 9. A catalyst
Options- A. initiates a reaction.
- B. lowers the activation energy of reacting molecules.
- C. is capable of reacting with any one of the reactants.
- D. can not be recovered chemically unchanged at the end of a chemical reaction. Discuss
- 10. For the irreversible elementary reactions in parallel viz
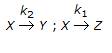 , the rate of disappearance of 'X' is equal to
, the rate of disappearance of 'X' is equal to
Options- A. CA(K1+K2)
- B. CA(K1 + K2)/2
- C. CA . K1/2
- D. CA . K2/2 Discuss
Chemical Reaction Engineering problems
Search Results
Correct Answer: Mixed reactor is always smaller than the plug flow reactor for all positive reaction orders for a particular duty.
Correct Answer: backmix reactor
Correct Answer: none of these.
Correct Answer: The space velocity of 3 hr-1 means that one third reactor volume of feed at specified conditions are being fed into the reactor.
Correct Answer: both (b) and (c).
Correct Answer: high pressure batch reactor.
Correct Answer: (atm)-1.(hr)-1
Correct Answer: increases
Correct Answer: lowers the activation energy of reacting molecules.
Correct Answer: CA(K1+K2)
Comments
There are no comments.More in Chemical Engineering:
Programming
Copyright ©CuriousTab. All rights reserved.
