Discussion
Home ‣ Chemical Engineering ‣ Chemical Reaction Engineering See What Others Are Saying!
- Question
For a reaction of the type,
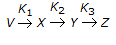 , the rate of reaction (- rx) is given by
, the rate of reaction (- rx) is given by
Options- A. (K1+K1)CX
- B. (K1+K2+K3)CX
- C. K1CV - K2CX
- D. (K1-K2)CX
- Correct Answer
- K1CV - K2CX
- 1. Wall thickness of schedule 40 pipe as compared to that of schedule 80 pipe is
Options- A. more
- B. less
- C. same
- D. cither (a) or (b); depends upon the I.D. of the pipe Discuss
- 2. Aniline point of the diesel is a measure of its __________ content.
Options- A. aromatic
- B. paraffin
- C. olefin
- D. naphthene Discuss
- 3. Dry powdery solid materials are transported by a __________ conveyor.
Options- A. belt
- B. bucket
- C. screw
- D. none of these Discuss
- 4. Size reduction does not occur due to compression in case of
Options- A. rod mills
- B. gyratory crushers
- C. jaw crushers
- D. smooth roll crushers Discuss
- 5. Thorium can be converted into U-233 in a __________ reactor.
Options- A. liquid metal cooled
- B. fast breeder
- C. thermal
- D. swimming pool Discuss
- 6. A rivetted joint does not fail by __________ of rivets.
Options- A. tearing
- B. shearing
- C. tearing of the plate across a row
- D. none of these Discuss
- 7. The synthetic fibres produced from __________ are known as rayon.
Options- A. lignin
- B. cellulose
- C. polyamides
- D. ethylene glycol Discuss
- 8. A/an __________ is used for changing the direction of a pipeline.
Options- A. elbow
- B. union
- C. flange
- D. disc compensator Discuss
- 9. Sucrose content in the raw juice extracted from sugar cane is about __________ percent.
Options- A. 1 - 2
- B. 15 - 20
- C. 50 - 60
- D. 80 - 85 Discuss
- 10. The ratio of volume of an atom to that of its nucleus is
Options- A. 1012
- B. 10-12
- C. 10-8
- D. 108 Discuss
More questions
Correct Answer: less
Correct Answer: aromatic
Correct Answer: screw
Correct Answer: rod mills
Correct Answer: fast breeder
Correct Answer: none of these
Correct Answer: cellulose
Correct Answer: elbow
Correct Answer: 15 - 20
Correct Answer: 1012
Comments
There are no comments.More in Chemical Engineering:
Programming
Copyright ©CuriousTab. All rights reserved.
