Discussion
Home ‣ Chemical Engineering ‣ Chemical Reaction Engineering See What Others Are Saying!
- Question
With decrease in temperature, the equilibrium conversion of a reversible endother-mic reaction
Options- A. decreases
- B. increases
- C. remains unaffected
- D. increases linearly with temperature
- Correct Answer
- decreases
- 1. NPK fertiliser is a __________ fertiliser.
Options- A. complex
- B. mixed
- C. nitrogenous
- D. phosphatic Discuss
- 2. Pick out the correct statement.
Options- A. In case of liquid-liquid extraction, no separation is possible, if the selectivity of the solvent used is unity.
- B. With increase in temperature, the selectivity of the solvent used in solvent extraction decreases.
- C. The selectivity of solvent used in solvent extraction is unity at the plait point.
- D. all (a), (b) and (c). Discuss
- 3. Reducing atmosphere is maintained in a
Options- A. calcination kiln
- B. blast furnace
- C. soaking pit
- D. L.D. converter Discuss
- 4. Crude oil is pumped by a __________ pump.
Options- A. gear
- B. centrifugal
- C. screw
- D. reciprocating Discuss
- 5. From pollution control point of view, the maximum permissible concentration of sulphur dioxide in atmospheric air is about __________ ppm.
Options- A. 1
- B. 5
- C. 50
- D. 500 Discuss
- 6. Except for monoatomic gases, the molal heat capacity at constant volume for all gases is __________ Kcal/Kg mole.° K.
Options- A. 3
- B. > 3
- C. < 3
- D. < 1 Discuss
- 7. The reaction A + B ? C has been conducted in a reactor as shown below.
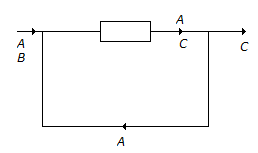
The number of balances (material) that can be made around the reactor are
Options- A. 1
- B. 2
- C. 3
- D. 4 Discuss
- 8. In classification, particles are said to be equal settling, if they have the same terminal velocities in the
Options- A. different fluids
- B. same fluid
- C. same field of force
- D. both (b) and (c) Discuss
- 9. __________ are used for the separation of coarse particles from a slurry of fine particles.
Options- A. Thickeners
- B. Classifiers
- C. Hydrocyclones
- D. Decanters Discuss
- 10. Polymerisation of poly functional monomers produces polymers having
Options- A. good machanical strength
- B. low viscosity
- C. low melting point
- D. none of these Discuss
More questions
Correct Answer: mixed
Correct Answer: all (a), (b) and (c).
Correct Answer: blast furnace
Correct Answer: centrifugal
Correct Answer: 5
Correct Answer: > 3
Correct Answer: 3
Correct Answer: both (b) and (c)
Correct Answer: Classifiers
Correct Answer: good machanical strength
Comments
There are no comments.More in Chemical Engineering:
Programming
Copyright ©CuriousTab. All rights reserved.
